Understanding the Intricate Link Between Obesity, Immunity, and the Microbiome
Written on
Chapter 1: The Multifaceted Nature of Obesity
Obesity is typically characterized by a body mass index (BMI) exceeding 30. However, this measurement can be contentious as it doesn't account for variations in body composition. For instance, many athletes with significant muscle mass may fall into the ‘obese’ category according to their BMI, despite being fit. A more accurate definition of obesity is the accumulation of excess body fat, with thresholds set at over 25% for men and 30% for women. In the United States, approximately 40% of adults are classified as obese, and this figure continues to rise.
While having some body fat is normal and even beneficial, excessive fat raises the risk of various health issues, including cardiovascular disease, type 2 diabetes, obstructive sleep apnea, certain cancers, osteoarthritis, and asthma. It is important to note that there are exceptions; some individuals classified as obese may still be in good health, while some who are not may face health challenges.
The modern environment does not facilitate healthy living. With easy access to high-calorie foods and sedentary lifestyles—spending around nine hours a day in front of screens—caloric intake often outweighs expenditure. This lifestyle is termed obesogenic, as it fosters conditions conducive to obesity.
The roots of obesity extend beyond mere overconsumption. Factors like genetics, gut microbiota, existing health conditions, and medications also play significant roles. While some individuals may be more predisposed to higher body fat levels, it does not mean they are destined for obesity; lifestyle adjustments are vital.
Moreover, the relationship between cause and effect in health and nutrition is rarely straightforward. For instance, while obesity influences gut microbiota, those same microbes can affect food metabolism and hunger levels, creating a complex interplay.
Section 1.1: The Relationship Between Obesity and Intestinal Immunity
Recent reviews have consolidated findings on the interplay between obesity and the gut microbiome, emphasizing the role of immune components within the intestines.
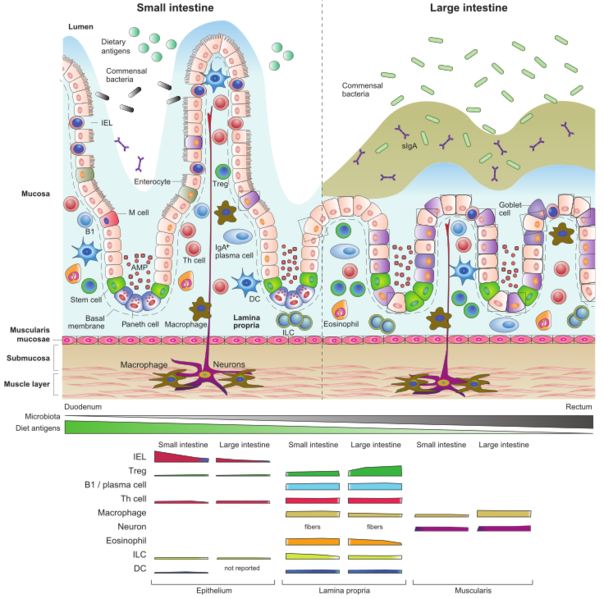
Key findings indicate that obesity triggers pro-inflammatory changes in intestinal immune cells, which are essential for the relationship between humans and their microbiota. These immune shifts can lead to increased metabolic inflammation, insulin resistance, and disrupted glucose regulation, creating a vicious cycle.
Obesity is often linked to dysbiosis, which refers to an imbalanced microbiome. Dysbiosis has connections to insulin resistance and dyslipidemia. In individuals with obesity, gut bacteria may produce harmful metabolites like trimethylamine N-oxide (TMAO), associated with cardiometabolic diseases. The microbiome's composition can also impact liver function and systemic glucose regulation.
Typically, our intestinal immune system regulates the microbiome, partly through immunoglobulin A (IgA), but obesity is associated with lower IgA levels. Conversely, gut microbes produce substances, such as short-chain fatty acids (SCFAs), that can influence immune responses, particularly promoting inflammation and impairing gut barrier function.
The narrative surrounding obesity is intricate, with numerous contributing factors and no simple solutions.
Chapter 2: Interventions and Future Directions
The first video titled "Obesity, Microbiome, and Cancer: Mechanistic Considerations" delves into the intricate connections between obesity, microbiota, and cancer mechanisms, highlighting potential pathways for intervention.
The second video, "Linking the Gut Microbiome, Obesity, and the Immune System," explores the relationships among gut microbiota, obesity, and immune function, emphasizing their interdependencies.
Lifestyle modifications, such as improved dietary habits and increased physical activity, can yield metabolic advantages, some of which may be mediated by changes in the gut microbiota and immune functions. Consuming dietary fiber can nourish beneficial microbes, potentially reversing some immune imbalances. However, transitioning to a high-fiber diet should be gradual to prevent gastrointestinal discomfort.
Probiotics and similar interventions raise questions about their effectiveness in reestablishing a healthy microbiome in obese individuals. Preliminary research suggests that fecal transplants from lean individuals to those with obesity might yield positive outcomes in animal studies. However, human trials are less promising.
Interestingly, specific probiotics, like Akkermansia muciniphila, have shown potential benefits for metabolic health, and innovative approaches such as genetically modified bacteria have demonstrated diabetes alleviation in rats, presenting intriguing possibilities.
In summary, understanding the signaling pathways between intestinal immune cells and gut microbes is crucial for pinpointing therapeutic targets to combat obesity and metabolic syndromes. As research progresses, insights into obesity-related intestinal dysfunction and immune mechanisms will pave the way for the development of effective and safe therapies.
Stay healthy, everyone!