Unlocking the Secrets of Atoms: How Scientists Capture Their Images
Written on
Chapter 1: Understanding Atomic Imaging
With the rapid advancements in technology, we are now able to visualize individual atoms, a feat that was once considered impossible!
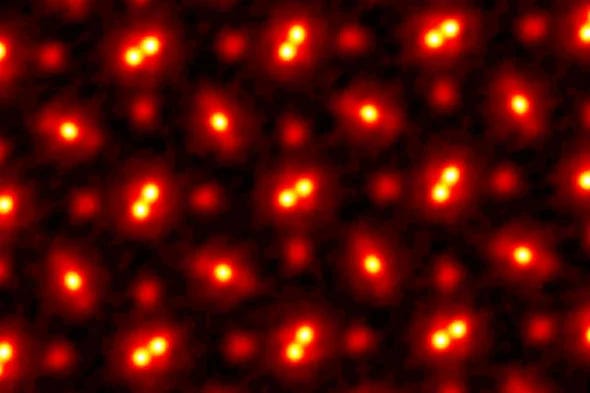
An illustration depicting atoms magnified 100 million times. (Credit: Cornell University)
When you snap a photo with your smartphone, it typically allows you to zoom in about ten times, depending on the device. For even closer views, a macro lens may be required. To observe micrometer-sized cells, light microscopes come into play. However, once we attempt to observe objects at the atomic scale, we hit a wall. The sizes of these particles are comparable to, or even smaller than, the wavelengths of visible light, resulting in diffraction that obscures our view. To circumvent this limitation, we turn to electrons.
Section 1.1: The Nature of Electrons
Quantum physics introduces some fascinating concepts, one of which is Wave-Particle Duality. This principle suggests that all matter behaves both as waves and particles simultaneously, meaning that every particle possesses a wavelength. Scientists harness this unique characteristic of electrons, which have shorter wavelengths than visible light, to obtain clearer images.

An electron microscope at work. (Credit: Dr. Graham Beards)
To achieve imaging at such a minuscule scale, researchers direct billions of high-energy electrons per second from an apparatus known as an electron gun. Utilizing magnetic lenses, these electrons are focused similarly to how optical lenses function in standard microscopes. Various techniques exist within electron microscopy, including Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and Scanning Transmission Electron Microscopy (STEM).
Section 1.2: Visualizing Atoms
Returning to our primary focus — the visualization of individual atoms. Atoms are incredibly diminutive, averaging just 0.1 nm in radius, which is significantly smaller than the wavelengths of visible light. However, electrons can achieve the necessary wavelengths for imaging. For example, the STEM method allows for atomic observation. The 'T' denotes transmission, meaning the detector captures electrons that have not engaged with the atoms being examined. This results in a discernible 'shadow' of the atoms. The 'S' signifies scanning, indicating that rather than using a broad electron beam, a tightly focused one is employed, tracing the sample much like an early CRT TV.
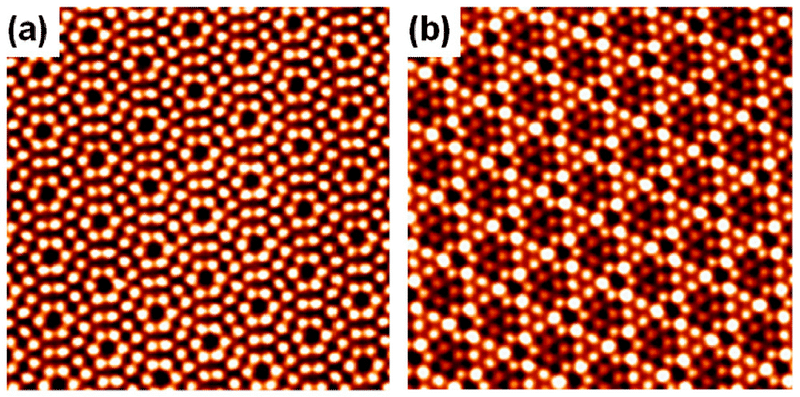
Additional images of atoms captured through advanced techniques. (Credit: Yeliang Wang)
In a groundbreaking achievement in 2021, Cornell University researchers set a Guinness World Record by magnifying an atom by 100 million times. They employed a technique called Electron Ptychography, which detects the position and angle of each electron. Although this method was conceived decades ago, technological limitations hindered its application. With the advent of modern machine learning and computing capabilities, scientists can now utilize this technique to delve deeper into the atomic world.
Chapter 2: Future Implications of Atomic Imaging
The implications of this high-resolution imaging technique are vast, especially concerning future electronic devices and batteries. However, there remain challenges to overcome. For this technique to be widely beneficial, it must be capable of accurately positioning individual atoms. While this is theoretically feasible, practical applications still need to be demonstrated. Nevertheless, the ability to observe individual atoms is an extraordinary accomplishment.
Here's how scientists take actual pictures of atoms - YouTube
This video delves into the groundbreaking methods scientists use to visualize atoms, explaining the intricate processes behind atomic imaging.
How to image atoms - YouTube
This video provides a detailed overview of the techniques employed to capture images of atoms, showcasing the technology and principles at play.
References